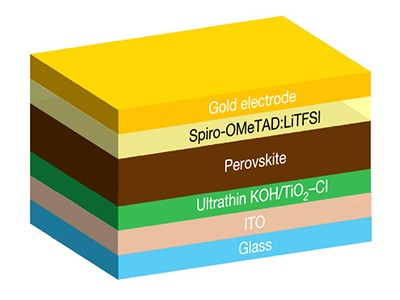
The next generation of solar cells could be based on materials called metal halide perovskites. Such devices are cheap, can be fabricated easily from solutions of the constituent materials and convert light into electricity with high efficiency (about 25.5% for cutting-edge devices1). Perovskite solar cells contain a film of an organic semiconductor, which is essential for achieving high power-conversion efficiency. But a key process in the preparation of these films, known as the doping step, typically takes many hours and generates by-products that seriously degrade solar-cell performance2. Writing in Nature, Kong et al.3 report a doping technique that might solve these problems, potentially overcoming a barrier to the commercialization of perovskite solar cells.
Doping — the deliberate addition of impurities — can modify the electronic properties of organic semiconductors. The discovery of this effect laid the foundation for the development of the multitude of electronic devices that pervade today’s society4. In perovskite solar cells, doped organic semiconductors are used to facilitate the transport of holes — positively charged quasiparticles produced as part of the cells’ power-generating mechanism. An organic semiconductor called 2,2′,7,7′-tetrakis (N,N-di-p-methoxyphenylamino)-9,9’-spirobifluorene (spiro-OMeTAD) is one of the most widely used hole-transporting layers (HTLs) in such devices.
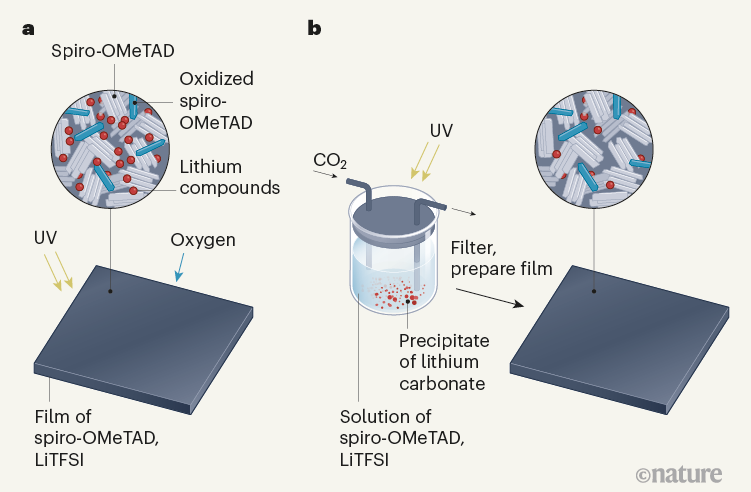
The conventional approach for doping spiro-OMeTAD HTLs is to use a compound called lithium bis(trifluoromethylsulphonyl)imide (LiTFSI) in combination with oxygen gas (O2). In this process, a pre-prepared spiro-OMeTAD film is irradiated by ultraviolet light and oxidized by oxygen in ambient air, assisted by LiTFSI (Fig. 1a)5. The oxidized product acts as the dopant that enables hole transport. Lithium oxides are also left in the film as by-products. Many perovskite solar cells made using this doping technique have achieved record-breaking power-conversion efficiencies6.
However, the process depends on the slow entry of oxygen into the film, and its subsequent diffusion through it — which usually takes between several hours and a day, depending on the ambient conditions. Furthermore, the lithium oxide by-products reduce the stability of the final solar cells7, because the lithium ions in the oxide insert themselves into the device’s perovskite layer (the ‘active’ layer of the device that absorbs light to produce charge carriers)8. Alternative doping strategies9 and dopant-free HTL materials10 have been developed to overcome these shortcomings, but none of the resulting solar cells has matched the power-conversion efficiency of the best perovskite solar cells. The conventional doping method therefore seemed to be a requirement for high power-conversion efficiencies.
Kong and colleagues’ innovation is to dope the spiro-OMeTAD before it is made into a film, by bubbling carbon dioxide through a mixture of the semiconductor and LiTFSI in solution under UV light (Fig. 1b). The UV light excites the semiconductor, whereupon the CO2 oxidizes it, forming a precipitate of lithium carbonate as a side product that can be removed by filtration. The filtered solution is then used to make an HTL that has a conductivity about 100 times higher than that of undoped spiro-OMeTAD, and approximately 3 times higher than that of HTLs made using the conventional doping method.
The authors found that perovskite solar cells made using CO2-oxidized spiro-OMeTAD have higher power-conversion efficiencies than those of devices produced using either non-doped or conventionally doped spiro-OMeTAD. More importantly, CO2 bubbling decreases the doping time to just one minute. Having such a short doping time will be essential for the commercial production of perovskite solar cells.
Because the lithium carbonate by-product is removed by filtration, the density of lithium ions in the resulting HTL is lower than that in HTLs made using conventional doping. Kong et al. show that solar cells prepared using their method are much more stable than are control devices prepared using conventional doping, consistent with the smaller amount of lithium in the HTL. The authors’ solar cells retain about 80% of their initial power-conversion efficiency after 500 hours of continuous operation, whereas the efficiency of the control devices rapidly drops to less than 75% after just 6 hours.
Even more excitingly, Kong and colleagues report that their CO2-bubbling method is not limited to small-molecule organic semiconductors such as spiro-OMeTAD — the conductivity of a broad range of polymeric organic semiconductors (mixed with LiTFSI) is between 2 and 100 times greater than that of polymer–LiTFSI films not treated with CO2. The power-conversion efficiency of perovskite solar cells made using the resulting doped polymeric HTLs is also substantially increased.
Perovskite solar cells show great promise, and have the potential to find industrial applications in the next few years. However, mass-manufacturing methods must first be developed that produce devices with long-term stability. This means that any compounds that reduce performance must be rigorously removed from HTLs during manufacturing. Kong and colleagues’ work is important because it shows the feasibility of removing stability-lowering compounds using potentially scalable doping methods. So, although the power-conversion efficiency and stability of the authors’ devices are not the best in the field, the new findings will inspire the development of other advanced doping strategies for rapidly producing clean films of organic semiconductors — thereby accelerating the pace of commercialization of perovskite solar cells.
"film" - Google News
June 02, 2021 at 10:22PM
https://ift.tt/3cc5DqU
Charge-carrying films for solar cells made quickly and cleanly - Nature.com
"film" - Google News
https://ift.tt/2qM7hdT
https://ift.tt/3fb7bBl
Bagikan Berita Ini














0 Response to "Charge-carrying films for solar cells made quickly and cleanly - Nature.com"
Post a Comment